Sponsors
Get Quality Data, Everytime
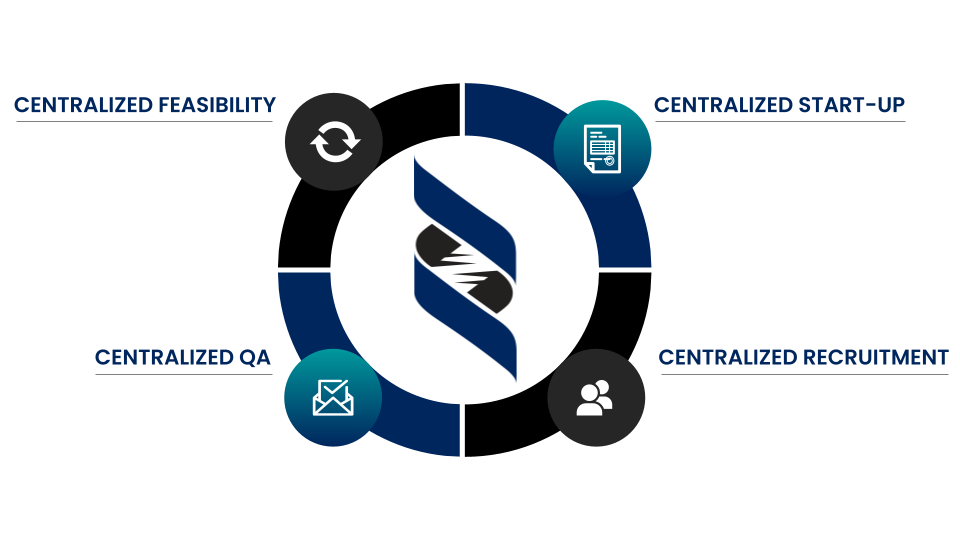
Committed to Becoming Your Long Term Site Network of Choice
At DelRicht Research, our paramount commitment is to ensure the highest standards of data quality and the expertise of our investigators. As a cohesive network of research sites, we unite seasoned investigators who possess a wealth of experience and a keen understanding of their respective medical communities. Each investigator within our network is carefully selected, embodying a dedication to precision and excellence in clinical research. They bring extensive expertise, honed through years of hands-on experience, and a profound understanding of diverse patient populations. Our cohesive approach allows us to synergize their expertise, fostering an environment where robust, reliable data is consistently generated.
In our quest for the advancement of medical science, we place an unwavering emphasis on the integrity and quality of data collected. Our network is built upon a foundation of meticulous data collection, management, and analysis, ensuring that every piece of information obtained is reliable, accurate, and ready for rigorous scrutiny. By combining the talents of our investigators with advanced data management practices, we cultivate an environment where data integrity is uncompromised. This commitment to data quality not only instills confidence in our sponsors but also fuels the continuous progression of medical research, contributing to the development of groundbreaking treatments and therapies that benefit patients worldwide.
-

COMMUNITY-BASED INVESTIGATORS
Amplifying Reach: Leverage Our Network of Dedicated Community Investigators
Partner with us to access a vast network of community-based investigators deeply embedded in their local communities, facilitating diverse and widespread trial participation.
-

EXPEDITED START UP
Accelerate Your Research: Streamline Trial Initiation with Our Expedited Startup Process
Choose DelRicht Research for a streamlined trial initiation process, reducing startup timelines and allowing for quicker deployment of your research initiatives.
-

RELIABLE RECRUITMENT
Optimize Enrollments: Ensure Reliable and Timely Recruitment through Our Expertise
Entrust us with your trial recruitment needs, and we’ll employ targeted strategies to ensure reliable participant enrollment, meeting your trial objectives effectively.
-

INSPECTION-READY DATA
Assured Quality: Access Inspection-Ready Data for Seamless Trial Monitoring
Collaborate with us to obtain reliable and inspection-ready data, allowing for smooth trial monitoring and regulatory compliance.
-

Community-based investigators
Mobilizing Local Expertise: A Vast Network of Community Investigators
DelRicht Research offers a strategic advantage by harnessing the power of community-based investigators who are deeply rooted in their local areas. Our extensive network comprises dedicated professionals who understand the specific needs and dynamics of their communities. Leveraging their established relationships, these investigators help bridge the gap between trial sponsors and potential participants. By tapping into this network, sponsors can significantly enhance trial outreach, engagement, and recruitment. We prioritize collaboration with investigators who possess a keen understanding of their community’s cultural, social, and healthcare landscape, ensuring trials are conducted with the highest level of relevance and rapport. Our investigators’ experience covers a variety of therapeutic areas which include:
- Internal Medicine
- Dermatology
- Psychiatry
- Neurology
- Urology
- Rheumatology












-

Expedited Start Up
Streamlined Onboarding: Reducing Time to Initiation
-

Reliable Recruitment
Targeted Strategies, Reliable Outcomes: Expert Trial Recruitment
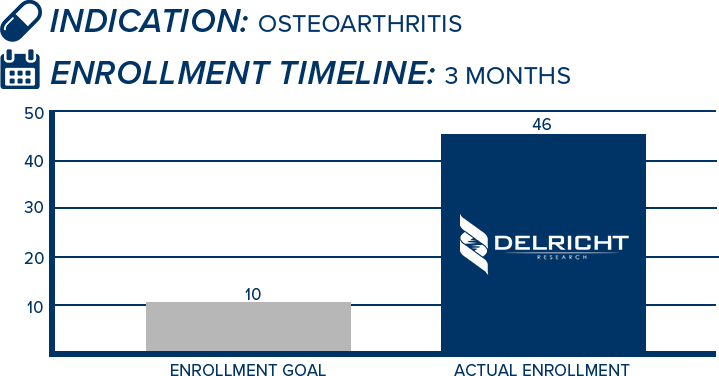
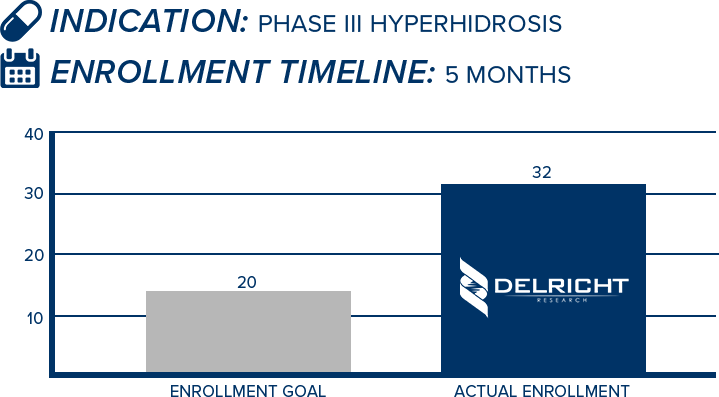
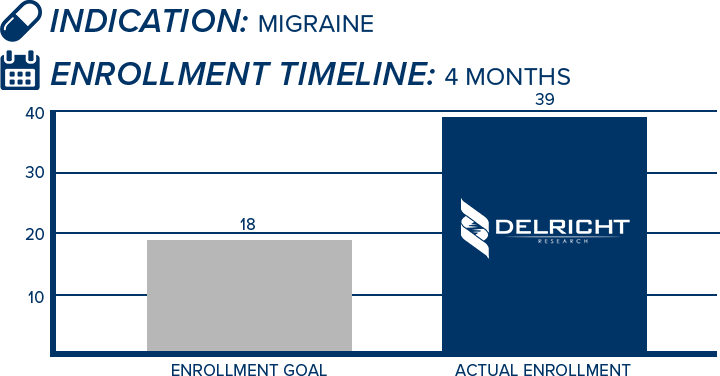
DelRicht Research specializes in reliable and targeted trial recruitment, leveraging our extensive experience and knowledge of diverse patient populations. We comprehend the significance of timely and accurate participant enrollment in meeting trial objectives. Our approach includes personalized recruitment strategies, utilizing insights from both our investigators and advanced analytics to identify the most suitable participants. We engage in proactive communication with potential participants, educating and guiding them throughout the process. This meticulous approach results in not only meeting recruitment goals but also in ensuring that participants are well-informed and committed, contributing to the overall success and quality of the trial.
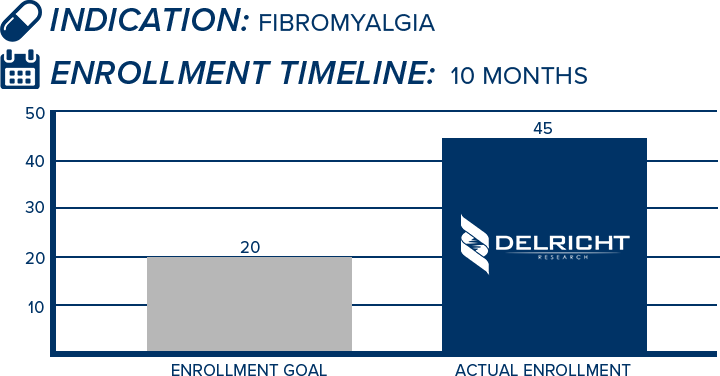
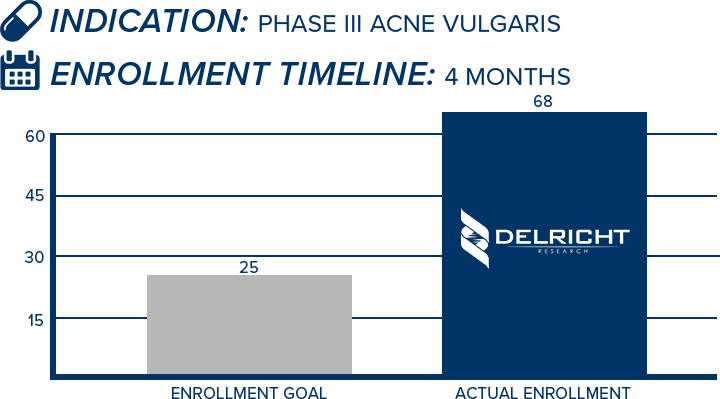
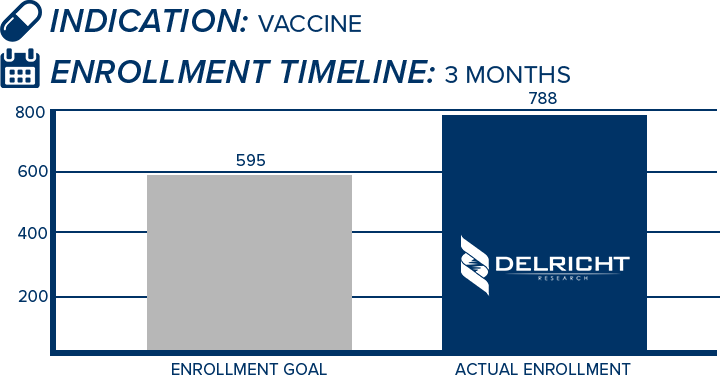



-

Inspection-Ready Data
A Commitment to Excellence: Data Prepared for Rigorous Inspection
DelRicht Research prioritizes the quality and integrity of trial data, recognizing its crucial role in the success of any clinical trial. We adhere to meticulous data collection, management, and analysis practices, ensuring that all data generated is of the highest standard and inspection-ready at any phase of the trial. Our emphasis on compliance and rigorous quality control measures guarantees that sponsors have confidence in the accuracy and reliability of the data, facilitating smooth trial monitoring, regulatory submissions, and eventual approval processes. By choosing DelRicht Research, sponsors can be assured that their trials are conducted with the utmost integrity, meeting the stringent demands of regulatory authorities and trial monitoring bodies.
